October 30, 2020
TissueTech Adds eKare’s Remote Wound Monitoring Solutions to its Host of Virtual Oversight Tools Used in Late-Phase Research of Biologic Candidate TTAX01 to Treat Complex Wagner Grade 3 and 4 Diabetic Foot Ulcers (DFUs)
Virtual technologies minimize risks associated with in-person patient visits, gives investigators the ability to monitor DFU wound healing in real-time via a telehealth platform
Miami, FL – October 29, 2020 –TissueTech, Inc., a pioneer in the clinical application of cryopreserved amniotic membrane and umbilical cord birth tissue allografts, announced today that they had selected the eKare inSight® 3D wound management system to accurately capture wound images and measurements in real-time after pivoting to a remote monitoring environment following the onset of the COVID-19 pandemic.
The eKare inSight® platform integrates with TissueTech’s existing suite of CFR part 11 compliant virtual technologies to further streamline and accurately document patient wound management data. Title 21 CFR Part 11 is a regulation that establishes the United States Food and Drug Administration regulations on electronic records and electronic signatures. In this case, with the eKare inSight® platform, investigators and their patients capture standardized wound measurements of complex Grade 3 and 4 DFUs at sites located throughout the U.S. Although the first trial was slated to start in March 2020, the pandemic forced TissueTech to delay the trial. Consequently, additional clinical solutions were adopted to support a remote monitoring environment and ensure data quality standards. As such, a risk-based monitoring approach was implemented – representing an important pivot for the organization.
“At the time, we did not know we would soon be dealing with the COVID pandemic,” said Tommy Lee, Vice President of Clinical Operations for TissueTech. “What made TissueTech adapt quickly was that we were not afraid to implement proven technologies to address the complexity of our clinical trials and associated data handling using the FDA Guidance on the Conduct of Clinical Trials during COVID-19 as a roadmap. In retrospect, we are happy we made the decision we did. We quickly adapted our clinical operations model when the pandemic hit, allowing us to get our pivotal phase 3 DFU program off the ground with minimal delays. In this case, we leveraged eKare’s expertise to meet the needs of our patients and our investigators.”
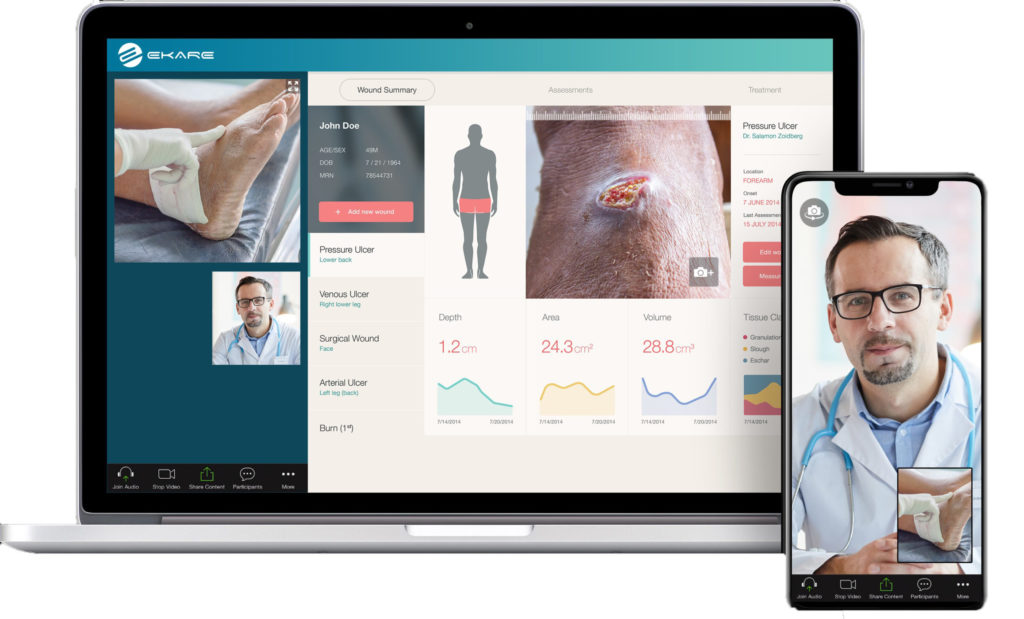
Trial investigators are using the eKare inSight® 3D wound management system as a tool to collect important wound data necessary to study the safety and efficacy of TissueTech’s cryopreserved umbilical cord biotherapeutic product TTAX01 to achieve complete wound closure of complex Grade 3 and 4 DFUs. These DFUs present with high-risk factors such as exposed bone, tendon, muscle, and/or joint capsule, and clinical suspicion of osteomyelitis. Patients may also use an eKare app on their smart device to privately capture and submit wound images, along with the generated measurements and analysis, to an encrypted, cloud-based portal in real-time for review by the investigator and sponsor.
“Now that COVID has caused the life science industry to adopt new patient-centric technologies, I believe there will be no going back,” said Amy Tseng, President, CEO, and co-founder of TissueTech. “Since our inception, TissueTech has witnessed an explosion of growth in the human birth tissue industry. This segment of the regenerative medicine market now generates over $1 billion annually.1 I believe it will continue to expand rapidly in the next few years. As an emerging biotechnology company, TissueTech will continue to set the standard for regenerative medicine.”
As a result of the COVID-19 pandemic, clinical trial teams need to further leverage remote monitoring technologies in certain situations. One example of this is when a patient can’t return to the clinic for a trial visit due to the risk associated with the virus. With the application of remote monitoring, both the patient and investigator will have a mechanism that allows for accurate, seamless data acquisition and calculation of wound measurements in real-time in a standardized, validated, and reproducible way.
“eKare is a leading provider in wound care imaging and measuring technology. We are proud to support TissueTech’s research,” said Kyle Wu, MD, Chief Medical Officer at eKare. “We are dedicated to providing sponsors, clinical research organizations, investigators, and their patients with the tools and technologies to facilitate improved outcomes through virtual visits and remote monitoring.”
About TissueTech, Inc.
TissueTech, Inc., the parent company of Amniox Medical, Inc. and BioTissue, Inc., is a scientific and market leader in the field of regenerative medicine. TissueTech manufactures a broad range of ocular, surgical, wound care, and soft tissue products marketed under these subsidiaries. Since the company’s inception, clinicians have performed more than 500,000 human implants of the company’s products and published more than 360 peer-reviewed studies supporting its platform technology. TissueTech is committed to an unwavering culture of integrity that places our patients’ safety and clinical outcomes above all else. Learn more at https://biotissue.com/.
About eKare
eKare is dedicated to the design and development of wound assessment solutions, including 3D wound dimensions and tissue classification capabilities, using the latest computer-vision and mobile technology. eKare’s innovative technology is creating new possibilities for improving delivery of wound care across the healthcare continuum, from inpatient hospital and skilled nursing facilities to ambulatory clinics and telemedicine. eKare’s mission is to advance the science and delivery of wound care by leveraging mobile and sensor technologies to connect patients, providers, and industry.
